Abstract
Introduction
Patients (pts) with systemic peripheral T/NK-cell lymphomas can have cutaneous involvement and their outcomes data are limited in the modern era. We aimed to retrospectively analyse pts who had T/NK-cell lymphomas with cutaneous involvement - PTCL-NOS or extranodal NK/T-cell lymphoma (ENKTL). We excluded cases if they ever had diagnosis of any Primary cutaneous T-cell lymphomas.
Methods
We conducted a retrospective study of pts with a histopathological diagnosis of T/NK-cell lymphoma established between 2002 and 2022 and identified from the Lymphoma Outcome Database at our center. Pts with a specific diagnosis of PTCL-NOS and ENKTL according to the WHO classification were included if they had biopsy proven involvement of skin. These pts were retrospectively assessed for demographic, clinical, laboratory data, pathology variables, response to treatment and survival outcomes. Fisher's exact test or Chi-square test was used to evaluate the association between two categorical variables. Kaplan-Meier method was used to estimate the time-to-event endpoints including progression-free survival (PFS) and overall survival (OS). Patients without progression or death were censored at the last follow-up date. Appropriate approval was gained from our institution's IRB.
RESULTS
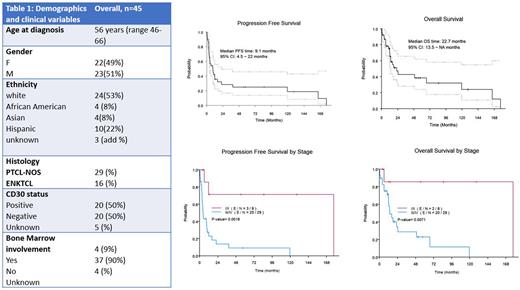
A total of 356 pts with cutaneous involvement by T/NK-cell lymphoma were analyzed and on further review 45 pts were found to meet the diagnosis of PTCL-NOS ( n=29) or ENKTL ( n=16) with histologically proven cutaneous/subcutaneous involvement according to WHO guidelines. The median follow-up time was 14.5 months (range: 1.84-128.6 months).
Twenty-three (51%) pts were men and 22 (49%) women. Median age at diagnosis was 56 years (range, 46-66), with 60% pts less than 60 years old. Thirty (70%) pts were diagnosed as having stage IV disease per Ann Arbor classification. Seven (28%) pts had involvement of an extranodal site other than skin. ECOG PS was 0 in 75% of evaluable pts. Among PTCL-NOS pts, 14/23 (48%) neoplasms were CD4+, 5 (17%) were CD8+; 5 were TCR-gamma+ and 6 pts were TCR-beta+. Of the samples evaluated, CD30 was expressed in 14/28 (50%) cases of PTCL-NOS and 6/12 (50%) of ENKTCL. Bone Marrow involvement was present in 10% of pts.
There was no standard frontline systemic therapy approach in this cohort. CHOP-based therapy was used in 15/29 (52%) pts with PTCL-NOS; 8/16 (50%) pts with ENKTL while received asparaginase base therapy. No pts received frontline radiation in the advanced stage setting.
Among the 33 evaluable pts in the cohort, the rate of OR to the 1st line treatment was 57.6% (95% CI: 39.2 ~ 74.5%). The rate of CR to the 1st line treatment was 54.5% (95% CI: 36.4 ~ 71.9%).
Among PTCL-NOS pts, 12/20 (60%) achieved CR, whereas in ENKTL pts 6/13 (46%) achieved CR to frontline treatment. Fifteen (30%) pts in the cohort had primary refractory disease. Seven pts with PTCL NOS had limited cutaneous/ subcutaneous involvement and received only skin directed therapy without systemic therapy. This pt subset had no evidence of progression, a behavior consistent with primary cutaneous PTCL-NOS. Advanced stage disease was the only variable that had an association with response ( p=0.004). In the whole cohort, the 2=year PFS was 28% with a median PFS of 9.07 (95% CI 4.5-21.98) months. For pts with stage I/II disease, the 2-year PFS was 71% ( 95%CI 0.45 - 1 ) with a median PFS of 177.4 months (95%CI -11.79 - NA). For pts with stage III/ IV disease, the 2-year PFS was 14% (95%CI 0.05 - 0.38 ) with a median PFS of 4.8 months (95%CI 3.19 - 11.99)(p=0.0018).
In the whole cohort, the 2-year OS was 47% with median OS of 22.67 ( 95%CI 13.53 - NA) months. For pts with stage I/II disease, the median OS was 177.37 months and for pts with stage III/ IV disease, the median OS was 13.63 months (95%CI 11.99 - NA) (p=0.0018 )
CONCLUSION: Cutaneous / subcutaneous involvement is an uncommon occurrence in pts with T/ NK cell lymphoma and is currently not incorporated in any prognostic scoring system. Systemic T/NK- lymphomas with cutaneous involvement have poor prognosis in our series and underscore the importance of considering skin biopsy in pts presenting with suspicious lesions. We hypothesize that the cutaneous/ subcutaneous tumor microenvironment creates an immune privileged site for chemo-refractoriness. A better understanding incorporating a combined modality approach and novel therapies are needed to improve the outcomes for these pts.
Disclosures
Nair:Incyte Corporation: Honoraria. Huen:kyowa: Consultancy. Ahmed:Merck: Research Funding; Myeloid Therapeutics: Consultancy; Seagen: Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Xencor: Research Funding; Chimagen: Consultancy, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees. Steiner:Seagen: Research Funding; BMS: Research Funding; GSK: Research Funding; Rafael Pharmaceuticals: Research Funding. Lee:Curio Science: Honoraria; Cancer Experts: Honoraria; Korean Society of Cardiology: Honoraria; Olson Research: Honoraria; Deloitte: Honoraria; Guidepoint Global: Honoraria; Janssen: Honoraria; Briston-Myers Squibb: Research Funding; Celgene: Research Funding; Octernal Therapeutics: Research Funding; Seagen: Research Funding; Takeda: Research Funding; Pharmcyclics: Research Funding; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Honoraria. Chihara:Eisai: Honoraria; AstraZeneca: Honoraria. Jain:Kite Pharma: Consultancy, Research Funding; Beigene: Research Funding; AstraZeneca: Research Funding; Aptitude Health: Membership on an entity's Board of Directors or advisory committees; Pharmacy Times: Membership on an entity's Board of Directors or advisory committees; Eli Lilly and Company: Consultancy, Honoraria. Srour:Orca Bio: Research Funding. Nieto:Secura Bio: Research Funding; Affimed: Other: Scientific advisory Board, Research Funding; Astra Zeneca: Research Funding. Nastoupil:ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Pinnix:Merck Inc: Research Funding. Neelapu:Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Pfizer: Consultancy, Honoraria, Other: Personal fees; Novartis: Consultancy, Honoraria, Other: Personal fees; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Calibr: Consultancy, Honoraria, Other: Personal fees; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Cellectis: Research Funding; Poseida: Research Funding; Bio Ascend: Consultancy, Honoraria; Aptitude Health: Consultancy, Research Funding; Medscape: Consultancy, Honoraria; Bluebird Bio: Consultancy, Honoraria; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Karus Therapeutics: Research Funding; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy. Flowers:Amgen: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; National Cancer Institute: Research Funding; Guardant: Research Funding; Burroughs Wellcome Fund: Research Funding; Ziopharm: Research Funding; EMD: Research Funding; Cellectis: Research Funding; Iovance: Research Funding; Janssen Pharmaceutical: Research Funding; Kite: Research Funding; Pfizer: Research Funding; Morphosys: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Xencor: Research Funding; TG Therapeutics: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Acerta: Research Funding; NPower: Current holder of stock options in a privately-held company; 4D: Research Funding; Spectrum: Consultancy; SeaGen: Consultancy; Pharmacyclics/Janssen: Consultancy; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Genentech/Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; BeiGene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding. Wang:Molecular Templates: Research Funding; Eastern Virginia Medical School: Honoraria; VelosBio: Consultancy, Research Funding; Loxo Oncology: Research Funding; Merck: Honoraria; Acerta Pharma: Honoraria, Research Funding; AbbVie: Consultancy; Milken Institute: Consultancy; Celgene: Research Funding; Vinverx: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Pepromene Bio: Consultancy; Dava Oncology: Honoraria; Genmab: Research Funding; Genentech: Consultancy, Research Funding; Oncternal: Consultancy, Research Funding; Lilly: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Physicians Education Resources (PER): Honoraria; OncLive: Honoraria; Moffit Cancer Center: Honoraria; MJH Life Sciences: Honoraria; Meeting Minds Experts: Honoraria; Medscape: Honoraria; LLC TS Oncology: Honoraria; IDEOlogy Health: Honoraria; Leukemia & Lymphoma Society: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Deciphera: Consultancy; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Practice Point Communications (PPC): Honoraria; Studio ER Congressi: Honoraria; Oncology Specialty Group: Honoraria. Vega:Geron Corporation: Research Funding; Allogene: Research Funding; Crisp Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal